Governance for Macquarie sponsored Investigator Initiated Clinical Trials (IICTs)
Overview and Process
A clinical trial at Macquarie University (MQ) is considered Investigator Initiated when the protocol is authored by Macquarie University investigators within the course and scope of their University appointment and the clinical trial addresses relevant clinical questions and not industry needs. Sponsorship of a clinical trial by the University is therefore considered and provided on a case-by-case basis. To determine whether the project is clinical research or a clinical trial, download the Clinical Research or Clinical Trial Decision Tool for assistance.
What is a clinical trial?
Clinical trial as per the ICG GCP definition is: Any investigation in human subjects intended to discover or verify the clinical, pharmacological and/or other pharmacodynamic effects of an investigational product(s), and/or to study absorption distribution, metabolism, and excretion of an investigational product(s) with the object of ascertaining its safety and/or effi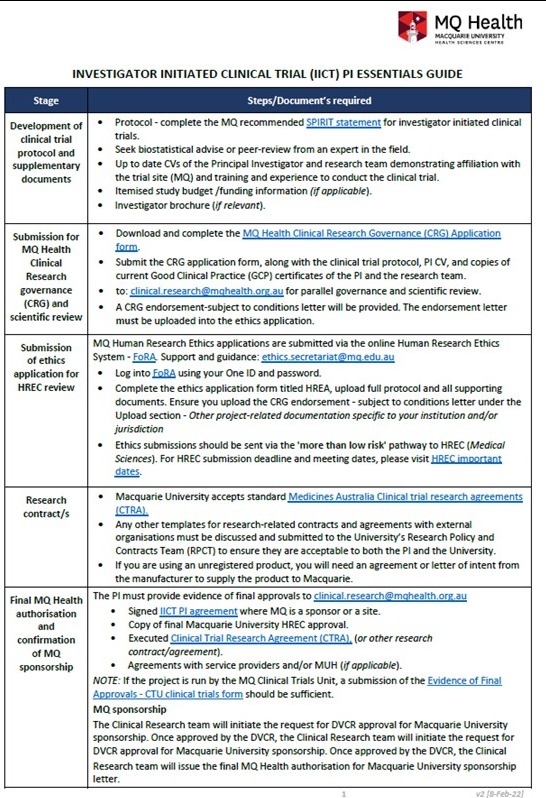 cacy. The terms clinical trial and clinical study are considered synonymous.
cacy. The terms clinical trial and clinical study are considered synonymous.
IICT Principal Investigator (PI) Essentials Guide
This high-level document outlines the stages of the IICT review and approval process at MQ and items to consider before, during and after approval of an IICT at Macquarie - IICT PI Essentials Guide.
Roles and responsibilities for IICTs
Macquarie University assumes the sponsor responsibility for clinical trials proposed by Macquarie University researchers. Some sponsor duties are delegated to the MQ Principal Investigator (PI) of the clinical trial, who takes overall responsibility for the conduct of the trial. However, accountability for all aspects of the trial resides with the trial sponsor.
researchers. Some sponsor duties are delegated to the MQ Principal Investigator (PI) of the clinical trial, who takes overall responsibility for the conduct of the trial. However, accountability for all aspects of the trial resides with the trial sponsor.
For detailed information on Macquarie University as a sponsor or a site and Principal Investigator responsibilities, please download the Investigator Initiated Clinical Trials where Macquarie University is a Sponsor – Institutional framework and guidelines brochure.
Clinical research governance review process and sponsorship assessment
The purpose of the MQ Health clinical research governance (CRG) assessment and scientific review is to:
- Determine whether the institution has the capacity and capability (including hospital and other resources, expertise, and participant pool) to carry out a specific clinical research/clinical trial within a given budget.
- Assess whether the proposed research project is in line with the Macquarie University strategic priorities.
- Assess the project for Macquarie University sponsorship.
- Ensure scientific review of the proposed research project.
- Ensure that the MQ Health Executive team is aware of and supports the proposed clinical research/clinical trial and whether there are any special insurance issues.
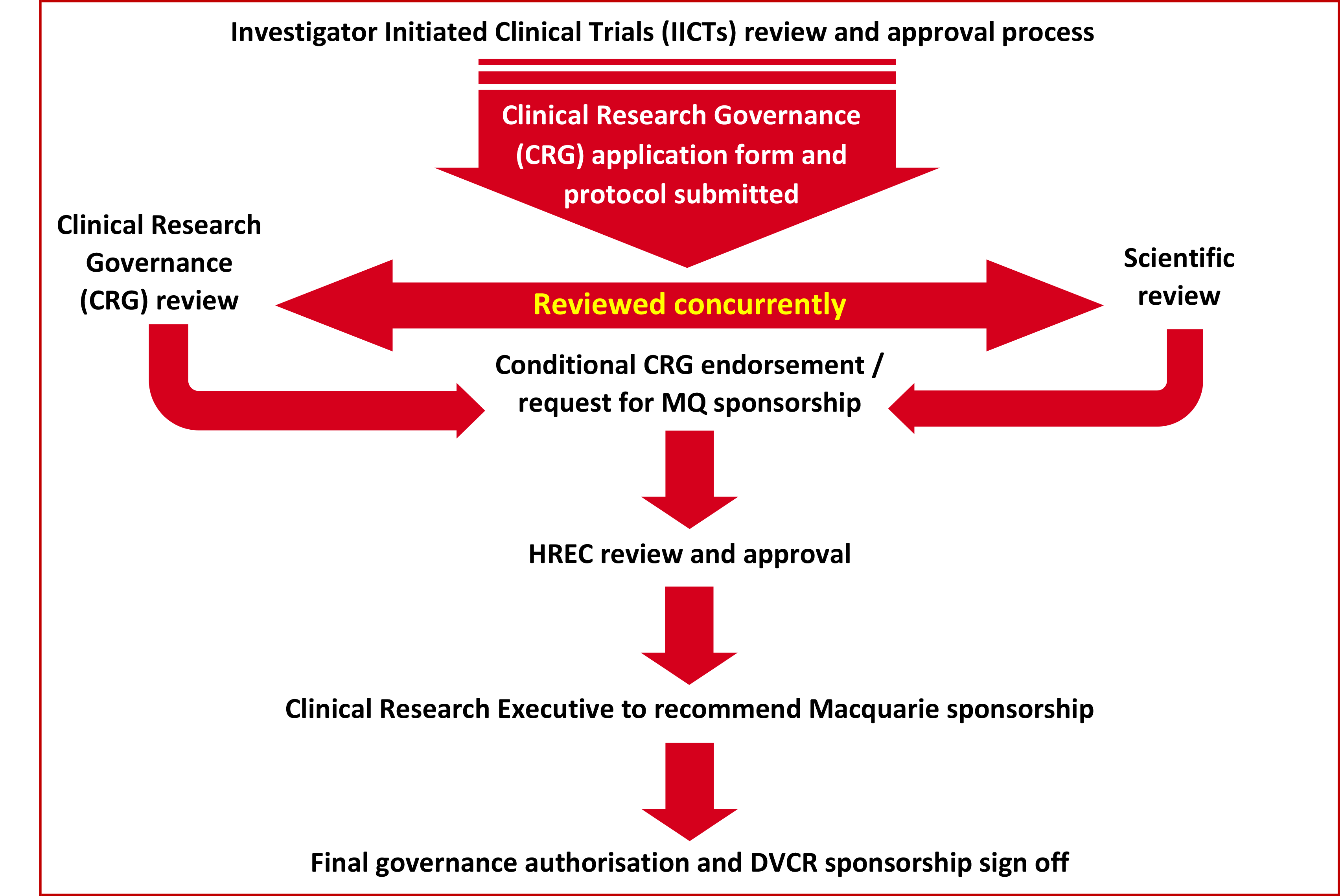
There are four steps in the Investigator Initiated Clinical Trial review process:
Step 1. Clinical trial protocol and supplementary documents development
Principal Investigator (PI) and the research team to develop a clinical trial protocol and supplementary documents for clinical research governance submission.
- Macquarie University recommends using the SPIRIT provides evidence-based recommendations for the minimum content of a clinical trial protocol. SPIRIT is widely endorsed as an international standard for trial protocols.
- PI to seek biostatistical advise or peer-review from an expert in the field to ensure quality of the research protocol.
- Up to date CVs of the PI and research team demonstrating affiliation with the trial site (MQ) and training and experience to conduct the clinical trial within the research area.
- Copies of current Good Clinical Practice (GCP) certificates of the PI and the research team. (Information about GCP in Australia and training at Macquarie can be found further down this page).
- Signed IICT PI agreement where MQ is a sponsor or a site.
- Itemised study budget/funding information.
- Investigator brochure (if applicable).
Step 2: MQ Health governance, scientific review, and conditional endorsement (concurrent review)
The PI completes the Clinical Research Governance IICT application form, obtaining all relevant signatures, then submit
- the Clinical Research Governance IICT application form,
- the clinical trial protocol,
- signed MQ PI agreement,
- copies of CVs for the PI and the research team,
- copies of current Good Clinical Practice (GCP) certificates for the PI and the research team,
- plus any any other supporting documentation relevant to the project e.g scientific review.
Submit your application one COMPLETE project submission package to: clinical.research@mqhealth.org.au. Incomplete submission will be returned to the PI and will require a complete re-submission of all documents.
- Reviewed by the Clinical Research Executive (CRE). (ref: IICT - Flowchart CRG process).
- Scientific review of the IICT where MQ is a sponsor - Internal review must be conducted by MQ Health Clinical Trials Scientific Review Panel in parallel with the CRG review. Includes review and advice on merit, scientific validity, and safety of the IICT by making recommendations to the PI and MQ Health CRE.
- Scientific review of the IICT where MQ is a site - External review is accepted. External review documentation must be submitted for CRE review.
Note: Investigator initiated clinical trials will be reviewed free of charge.
Timeline: IICTs are allocated for review at monthly CRE meetings. Outcomes will be sent to the PI within 2 to 3 working days of the meeting.
An initial governance endorsement letter will be emailed to the PI by the Clinical Research team, stipulating the conditions of the governance endorsement. These conditions must be satisfied and approved by the CRE prior to project commencement.
Note: The MQ Health governance assessment and initial endorsement confirm MQ Health's support of the project but are not the final authorisation to start the project.
Step 3: Ethics, insurance, and research contracts
PI prepares and submits the following documents
Human Research Ethics submission
- Human Research Ethics applications are managed via the online MQ Human Research Ethics System - FoRA.
- Log into FoRA using your One ID and password.
- Complete the ethics application form titled "HREA", upload the full protocol and all supporting documents.
- Upload the "CRG endorsement - subject to conditions" letter under the upload section.
- Ethics submissions should be sent via the 'more than low risk' pathway to HREC (Medical Sciences). For HREC submission deadline and meeting dates, please visit HREC important dates.
For further information visit MQ Human Research ethics or contact: ethics.secretariat@mq.edu.au for support and guidance.
Insurance
Any investigator initiated clinical trial where Macquarie University is a sponsor or a site, the project has been approved by the MQ HREC, and final governance approval has been granted, is covered by Macquarie University Clinical Trials insurance policy. The MQ Clinical Trials insurance policy does not cover external sites. Please note: specific categories of clinical trials will need to be referred to the insurer via the Office of Risk and Assurance. Please contact the Clinical Research Manager if your clinical trial involves any of the following categories:
- Trials involving pregnant women
- Trials involving children 5 years of age or less
- Trials involving Blood Plasma and whole blood products
- First in Human Phase 1 trials
- Any trial which is to be undertaken outside of Australia. Some countries require local insurances, and the insurer may arrange cover if a specific request is made and a fee of approximately $AUD 4,000 plus fronting fees will be charged.
Research Contract/funding agreement
- Macquarie University accepts standard Medicines Australia Clinical trial research agreements (CTRA).
- Any other templates for research-related contracts and agreements with external organisations must be discussed and submitted to the University’s Research Policy and Contracts Team (RPCT) to ensure they are acceptable to both the PI and the University.
- Detailed information about the process, the timeframes for funded and non-funded research agreements and key contacts can be found on the RPCT webpage.
- Note: Only the Deputy Vice-Chancellor (Research) or his delegate is authorised to sign research contracts or agreements on behalf of Macquarie University.
Service provider approvals/agreement/s
- PI to seek relevant service provider agreements – e.g. Macquarie Medical Imaging (MMI), pathology, MUH pharmacy.
Step 4: Final governance authorisation and confirmation of Macquarie sponsorship
The Clinical Research Executive or MQ Health Executive provides final governance authorisation, and recommendation for Macquarie University sponsorship to the Deputy Vice-Chancellor research for approval before the project may commence.
The PI must provide evidence confirming that the following conditions outlined in their endorsement letter is in place:
- Final Macquarie University HREC approval letter
- Executed Clinical Trial Research Agreement (CTRA), (or other research contract/agreement)
- Agreements with service providers and/or MUH (if applicable).
NOTE: If the project is run by the MQ Clinical Trials Unit, a submission of the Evidence of Final Approvals - CTU clinical trials form should be sufficient.
The Clinical Research team will initiate the request for DVCR approval for Macquarie University sponsorship. Once approved by the DVCR, the Clinical Research team will issue the final MQ Health authorisation for Macquarie University sponsorship letter.
Timeline: 5 working days
Good Clinical Practice (GCP) Training
Good clinical practice (GCP) is the international standard for conducting clinical research. Accredited GCP training is expected by all members of the research team involved in an investigated clinical trial where Macquarie University is a sponsor or a site.
Researchers must design their clinical trial to ensure that it meets the requirements of GCP in addition to the National Statement and other relevant guidelines. The GCP guidelines apply to clinical research and detail the requirements for trial documentation, protocol amendments, requirements such as indemnity, reporting lines for adverse events and provision of medical care for trial participants.
Researchers in Australia are required to have adequate experience, qualifications or be supervised under the Australian Code for the Responsible Conduct of Research, 2018, National Statement and the ICH Good Clinical Practice guidelines.
Further information can be found on the MQ Human Research ethics clinical trials webpage.
Good Clinical Practice (GCP) Certification at Macquarie University (accredited ICH GCP training)
GCP training is now offered through the MQ Clinical Trials Unit. Macquarie University has adopted the minimum standards defined by TransCelerate Biopharma Inc to recognise GCP training courses that contain material meeting the minimum criteria agreed to by its member organisations.
Macquarie University Clinical Trials Unit (CTU) offers one-day TransCelerate accredited GCP training free of charge, twice a year. The training is delivered as an “introduction” to GCP (5-6 hours) or a “refresher” training session (1.5-2 hours) for staff who just need to update their qualifications.
GCP training sessions
This TransCelerate accredited GCP training session is tailored for all staff involved in clinical research. The training is dynamic and interactive and gives participants an in-depth understanding of GCP and how it relates to everyday research activity. Led by specially trained CTU staff members in partnership with Sophie Mepham GCP™ and Praxis Australia. The training will be delivered over 5-6 hours. GCP Certificate will be provided to all participants on successful completion of the training (TransCelerate accredited).
Introduction to GCP training workshop - 2024
Date: Thursday 15 Feb 2024
Time: 9am - 1pm
Location: Continuum Room, Level 3, 75 Talavera Road, Macquarie University NSW 2109
To register your interest, or for further information on GCP training please contact: clinicaltrials@mq.edu.au.
Please note that places are limited, and sessions may be subject to change. GCP training outside these dates can be arrange directly with the CTU. Please note: this training will incur a fee.
Please note: Macquarie University does not mandate you gain GCP certification through the CTU. Alternative GCP training is offered through many companies who have self-attested and courses meet the TransCelerate GCP Training Minimum Criteria. Macquarie University endorses using the Good Clinical Practice (ICH GCP) Training Course - Genesis Research Services. This online course is $10 and is designed to define the Minimum Criteria for Good Clinical Practice (GCP) training of investigators and site personnel (based upon ICH E6 R2).
For further information on other externally recognised training providers, visit the TransCelerate Biopharma- GCP Providers webpage.
Clinical Trial Notification (CTN) or Clinical Trial Approval (CTA) submission to the Therapeutic Goods Administration (TGA)
Clinical Trial Notification (CTN) scheme is a notification process. The clinical trial sponsor must notify TGA of the intent to sponsor a clinical trial involving an 'unapproved' therapeutic good. This must take place before starting to use the goods. The notification form (eCTN) must be submitted online and accompanied by the relevant fee.
eCTN submission to the TGA is required if:
- A product for the trial is not entered on the Australian Register of Therapeutic Goods (ARTG), including any new formulation of an existing product or any new route of administration; or
- The proposed use of a registered or listed product is outside the conditions of its marketing approval.
eCTN submission if Macquarie University is the sponsor:
- The eCTN submission for Macquarie University is done by the Macquarie University Clinical Research Manager.
- Macquarie University has an institutional account with TGA and an authorised person to be the institutional administrator.
- You need to liaise with the Clinical Research Manager or +61-2-9850-2834 and supply the full project details, including all site details before the eCTN application can be lodged at the TGA portal.
- Please note that HREC approval is required before CTN submission.
- Credit card payment of the CTN fee is preferable.
- For further information, contact the Clinical Research Manager or +61-2-9850-2834.
Clinical Trial Approval (CTA) (formally Clinical Trial Exemption (CTX) scheme) is an approval process. The sponsor submits an application to TGA seeking approval to supply 'unapproved' therapeutic goods in a clinical trial. The application must be accompanied by the relevant fee.
The TGA evaluates summary information about the product including relevant, but limited, scientific data (which may be preclinical and early clinical data) prior to the start of a trial.
The HREC is responsible for considering the scientific and ethical issues of the proposed trial protocol.
CTA applications are submitted using paper-based forms. There are two forms that must be completed by the sponsor and submitted to TGA via post.
More information about CTN and CTA schemes is available on the TGA website.
Initiation and Management
Clinical Trial Initiation
Clinical Trial Registries
Clinical trial registries allow you to find clinical trials in Australia. A registry is defined as an organisation or website that either:
- lists clinical trials being conducted (or that have recently been conducted) in Australia (or internationally, including Australia).
- provides a mechanism for patients or others to register their interest in participating in an Australian clinical trial.
- provides a link between potential participants and Australian clinical trials.
The two main registries are:
- Australian New Zealand Clinical Trials Registry (ANZCTR) - is a register of clinical trials being undertaken in Australia and New Zealand.
- ClinicalTrials.gov - is a US site listing clinical trials in the US and in other countries, including Australia.
For information on other clinical trial registries visit Australia Clinical Trials Registries.
Other trial registration information
International Committee of Medical Journal Editors (ICMJE) - Policy on Trial Registration
ICMJE requires, and recommends that all medical journal editors require, registration of clinical trials in a public trials registry at or before the time of first patient enrolment as a condition of consideration for publication.
Clinical Trial Management
The TGA: ICH Guideline for Good Clinical Practice (GCP) states that the PI is responsible for managing quality throughout all stages of the clinical trial. It emphasises the importance of a risk-based approach to quality management. One element of this is risk management where resources are focused on reducing and managing significant risks to participants, including rights, well-being, and safety, as well as the reliability of the trial results.
Trial Risk Assessment and Management
What is a risk?
A risk is defined as the effect of uncertainty on objectives. A risk is often assessed in terms of a combination of the consequences of an event and the associated likelihood of occurrence.
What is risk management?
It is a proactive approach to preventing potential issues or harm with the aim of avoiding unwanted outcomes and involves the following components:
- Identify and assess the risks
- Mitigate the risks
- Review and monitor risks
- Communication and documentation of risks
- Continuous evaluation of risks
Managing risks during the clinical trial life cycle
Risks may be identified at different stages of the clinical trial life cycle. These risks may be new, or pre-existing but will change over the course of the trial. Risks can be mitigated through continuous monitoring and evaluation improving the overall risk management of the trial.
Who is responsible for managing risk in a clinical trial?
The Principal Investigator is responsible for evaluating all risks to participants rights and the reliability of the results, trial conduct, design and methods before the trial commences. They are responsible for developing and implementing a plan to control these risks to an acceptable level.
Other guidance and reading
Risk-based management and monitoring of Clinical Trials involving therapeutic Goods
ICH E6 R2 – Best Practices for Implementing A More Formal Risk Management Process
Clinical Leader| Article | May 31, 2019 - Dr. Volker Hack, Executive Director, Process & System Optimization, and Brian Barnes, Director, Clinical Management, PPD
Safety Monitoring and Reporting
The Australian sponsor or Principal Investigator of a clinical trial is responsible for safety reporting. The Macquarie University HRECs have adopted the NHMRC Guidance on safety monitoring and reporting in clinical trials involving therapeutic goods which addresses the monitoring, collection and reporting of adverse events and adverse reactions that occur in clinical trials involving investigational medicinal products and investigational medical devices for trials conducted under the Clinical Trial Approval (CTA) or Clinical Trial Notification (CTN) schemes. The Guidance is also broadly applicable to all clinical trials involving therapeutic goods.
Supplementary guidance is also available:
- Risk-based management and monitoring of Clinical Trials involving therapeutic Goods
- Reporting of Serious Breaches of Good Clinical Practice (GCP) or the Protocol for Trials Involving therapeutic Goods
- Data Safety Monitoring Boards
MQ REPORTING REQUIREMENTS
- Regular reporting to Clinical Research Executive if specified in the approval letter and the HREC (annually as part of HREC standard reporting).
- Serious Adverse Events, SUSARs and USMs reports sent to MQ Clinical Trials Safety Officer within 24 hours at ResearchSafetyReporting@mq.edu.au, and cc. clinical.research@mqhealth.org.au using the safety report form.
Data Management
- Use of MQ REDCap for managing sensitive health data. REDCap includes data and project management features, as well as ethics safeguards unavailable in other platforms. MQ REDCap is maintained internally at Macquarie University and all data resides in NSW.
- Join a MQ Yammer group to seek out internal user community support. Yammer is a private, university wide communications and social network platform which allows you to communicate and collaborate with online colleagues online at your desk or on the go.
Data Safety Monitoring Board (DSMB) guidance
If determined by the CRE; the research team might need to establish a DSMB for the study. Macquarie University has developed a DSMB guidance for PIs. For further information on DSMB’s visit the Resources, Date and Guidance tab.
Resources, Dates and Guidance
Data Safety Monitoring Board (DSMB) guidance
- Data Safety Monitoring Board (DSMB) is an independent and multidisciplinary group established to provide oversight of investigator initiated clinical trials (IICTs) of which Macquarie University is a sponsor. This includes clinical trials which include non-pharmacological, behavioural and lifestyle interventions.
- The DSMB will review, at intervals, accumulating clinical trial data, in order to monitor the progress of the trial and to make recommendations on whether to continue, modify or stop the trial for safety or ethical reasons.
- The duration of the function of individual DSMBs should be stated in the protocol and could vary from study to study depending on the requirements and key time points in the study.
- IICT - Data Safety Monitoring Board (DSMB) - Terms of Reference (TOR)
Research Principles and Guidelines
In Australia, all research involving humans must comply with the principles set out in the following guidelines:
- National Statement on Ethical Conduct in Human Research (2007) — updated 2018 (NHMRC)
- Australian Code for the Responsible Conduct of Research
- Applicable State and territory guidelines
Clinical trials of medicines and medical devices also must comply with:
- ICH Guideline for Good Clinical Practice (ICH/E6(R1), E6(R2) Therapeutic Goods Administration) Replaces: Note for guidance on good clinical practice (CPMP/ICH/135/95)
Clinical trials of medical devices must also comply with:
- ISO 14155:2020 Medical devices - Clinical investigation of medical devices for human subjects: Good clinical practice.
To fulfil the prospective registration requirement, i.e. obtain registration number (ACTRN) prior to enrolment of the first participant, we recommend that Australian and New Zealand registrants commence the registration process at least three weeks prior to the anticipated recruitment start date. All other registrants should allow substantially longer.
Australian Clinical Trials – resources for researchers
AustralianClinicalTrials.gov.au is a joint initiative between the National Health and Medical Research Council and the Department of Industry, Innovation and Science to provide information and resources to consumers, health care providers, researchers, and industry about clinical trials
TGA - Australian Clinical Trials Handbook
Provides guidance on the legislative, regulatory, and good clinical practice (GCP) requirements when conducting clinical trials in Australia using 'unapproved' therapeutic goods. It assists trial sponsors, Human Research Ethics Committees (HRECs), investigators and approving authorities (institutions) to understand their roles and responsibilities under the therapeutic goods legislation.
ICH Good Clinical Practice Guideline
An internationally accepted standard for the designing, conducting, recording and reporting of clinical trials.
Clinical investigation of medical devices for human subjects - Good clinical practice (ISO 14155)
For medical devices - addresses good clinical practice for the design, conduct, recording and reporting of clinical investigations carried out in human subjects to assess the safety or performance of medical devices for regulatory purposes.
Suprin, M et al. Quality Risk Management Framework: Guidance for Successful Implementation of Risk Management in Clinical Development, Therapeutic Innovation & Regulatory Science 2019, Vol. 53(1) 36-44
US FDA: A Risk-Based Approach to Monitoring of Clinical Investigations Questions and Answers
National Institute for Health Research: Clinical Trials Toolkit, Risk Assessment This document clarifies the responsibilities of those involved in clinical trials to monitor and report adverse events and other safety issues.
Consolidated Standards of Reporting Trials CONSORT (Encompasses various initiatives developed by the CONSORT Group to alleviate the problems arising from inadequate reporting of randomized controlled trials, e.g. SPIRIT protocol).
Further reading: Guidance on maximising the impact of qualitative research in feasibility studies for RCTs was developed to help researchers consider the full range of contributions that qualitative research can make in relation to their particular trial. O’Cathain, A., Hoddinott, P., Lewin, S. et al. Maximising the impact of qualitative research in feasibility studies for randomised controlled trials: guidance for researchers. Pilot Feasibility Stud 1, 32 (2015).
Training and Learning Modules
Australian Clinical Trials currently have three eLearning modules available:
- Clinical Trials Environment in Australia: This module provides an overview of how clinical trials work in Australia.
- Ethical Issues related to Clinical Trials: This module looks at the ethical aspects of clinical trials and how to manage the process of an ethics review.
- Research Governance related to Clinical Trials: This module looks at the research governance process including all the checks and clearances that are necessary to start a research project.
- PRAXIS training: workshops include Clinical Trial Essentials
- ARCS Australia Professional Development in Therapeutics
Other institutions resources
The VCCC has partnered with the Melbourne Academic Centre for Health (MACH), and with assistance from the Melbourne Children's Trials Centre, to develop a suite of resources to support researchers conducting Investigator-initiated trials to use and/or adapt to meet the needs of their trial or institution.
