National Statement (2023)
The National Statement on Ethical Conduct in Human Research
Read the statementThe National Statement on Ethical Conduct in Human Research
Read the statementRead our Human Research Ethics Policy
View policyFriday 16 August 2024

Over the past month, we have been advising researchers on the upcoming integrated HREA form which will go live on Tuesday 20th August. The new form is essentially made up of 3 sections
Introduction to new Exempt and Risk Assessment workflow
While the DMP and HREA sections remain largely unchanged, the 2023 updates to the National Statement introduce new requirements for risk assessment in human-focused studies. Last week, we provided MQ researchers with an overview of the relevant pathways. This week, we will introduce the new Exempt and Risk Assessment workflow (Figure 1), along with how-to guides, instructional videos, and opportunities to attend drop-in sessions (Table 1) for one-on-one support and advice
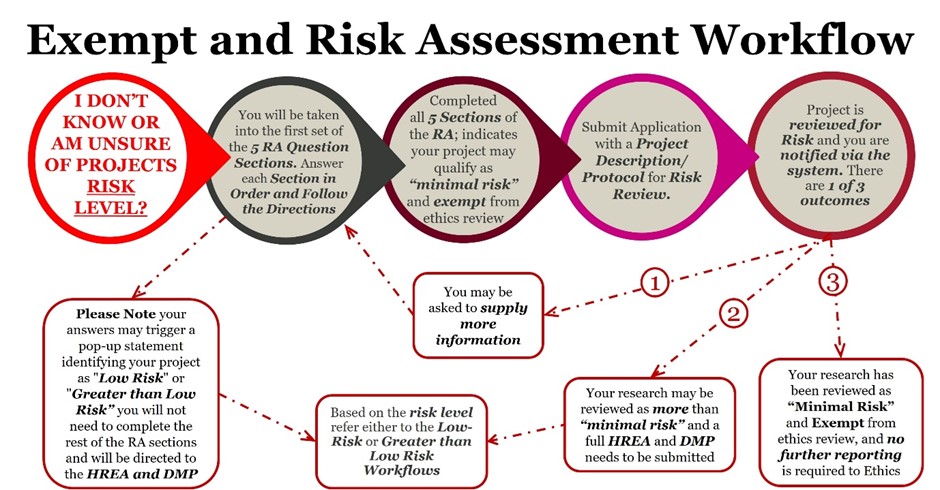
Figure1: The new Exempt and Risk Assessment Workflow – detailing the process from submission to either exemption from ethics review or determination of risk level
Guides and Drop-ins
To help you navigate your way through the new Risk Assessment and DMP filter questions of the HREA, we have created how-to-guides and instructional videos:
We will be holding drop-in sessions to assist with submissions of the new integrated Human Research Ethics Applications (HREA). These sessions will run from 11 am to 1 pm and are informal and flexible, allowing you to join at any time within the two-hour window and stay as long as you need. Representatives from the Ethics Secretariat and, in some cases, the Research Data Management teams will be available. Sessions will be held either in person in the Senate Room (16WW 127 Senate Meeting Room) or virtually.
Please register via MyRDC, to receive the meeting details in your calendar. Please refer to Table 1 for the dates, delivery and section advice available on the day.
Please bring your laptop if you require assistance with online submissions, as well as to access our instructional guides and videos.
Table 1: Date, Day and Location of the Drop In Sessions – ALL sessions will be held between 11am – 1pm.
DATE | DAY | SECTION ADVICE | DELIVERY |
13/11/2024 | Wednesday | RA and DMP | Virtual |
20/11/2024 | Wednesday | RA and DMP | Virtual |
20/11/2024 | Wednesday | RA and DMP | Virtual |
27/11/2024 | Wednesday | RA and DMP | Virtual |
04/12/2024 | Wednesday | RA and DMP | Virtual |
11/12/2024 | Wednesday | RA and DMP | Virtual |
If you have any questions in the meantime or would like to organise a presentation or workshop session for your research group, please don't hesitate to reach out.
Human Research Ethics Team: ethics.secretariat@mq.edu.auResearch Data Management Team: research.data.management@mq.edu.au
Friday 9 August 2024

Last week we provided more information about the new unified Human Research Ethics Application (HREA) form which integrates the new Risk Assessment questions and the Data Management Plan. Specifically, how the addition of the Risk Assessment questions are designed to conform to the new risk continuum updated in The National Statement on Ethical Conduct in Human Research(2023), and how this integration impacts Macquarie’s Data Management Plan (DMP) questions. This week we will provide more background information and an overview of the pathways involved.
Who completes the Risk assessment questions?
The MQ Filter questions at the start of the HREA form now look a little different when starting a new project. After confirming your project title and entering the CI’s information you will be asked: Do you know the risk level and review pathway of your project?
You do not have to complete the Risk Assessment questions. Just confirm you know the risk level of your project and complete HREA as normal.
You will also need to complete the DMP section at the end of the form.
You will be taken into the first set of Risk Assessment question sections.

Figure 1: Workflow of Human Research Ethics review pathways
How does the Risk Assessment Section work?
The Risk Assessment questions are organized into 5 sections to identify any foreseeable risks, discomfort, or specific types of data collection associated with your research. You will only see all 5 sections if none of your answers indicate that your project is above "Minimal Risk." If at the completion of a section it determines that your project is "Low Risk" or "Greater than Low Risk," you will receive a pop-up statement in red informing you of the assessed risk level. You will not need to complete the rest of the Risk Assessment sections and will then be directed to the main HREA and integrated DMP form for completion.
Do I still have to do a DMP if my research is Exempt?
If your project has been assessed as exempt from needing an ethics review, you may still need to complete a Data Management Plan (DMP). This could be required for several reasons, such as part of your candidature, a requirement from your funding body or publishing journal, or an institutional mandate. It's important to check these requirements yourself. You can find more information on the Research Data Management SharePoint or contact the team directly via the email address below.
Next week, we'll share more information about Minimal Risk research and research that may be Exempt from ethics review, including a detailed outline of the Risk Assessment and Exempt pathways. In the following weeks, we will provide you with how-to guides, options for drop-in sessions, and informative walkthrough videos. If you have any questions in the meantime or would like to organize a presentation or workshop session for your research group, please don't hesitate to reach out.
Human Research Ethics Team: ethics.secretariat@mq.edu.au
Research Data Management Team: research.data.management@mq.edu.au
Friday 26 July 2024
Recently we advised you of the new unified HREA form which integrates the new Risk Assessment questions and the Data Management Plan. Below is some background information and the reason behind these updates.
The addition of the Risk Assessment questions are designed to conform with the updates to The National Statement on Ethical Conduct in Human Research (2023). Relevant to the new requirements to assess risk in all human research projects is the transition from distinct risk categories to a risk continuum.
Previously, risk categories were defined as:
These have now been replaced by a continuum ranging from high risk to minimal risk. A key feature of this change is the introduction of the "Minimal Risk" category. If your project falls into this category, it may be exempt from ethics review.

Lower Risk | Higher Risk | ||
Minimal | Low | Greater than low | High |
No risk of harm or discomfort; potential for minor burden or inconvenience | No risk of harm; risk of discomfort (+/- foreseeable burden) | Risk of harm (+/- foreseeable burden) | Risk of significant harm (+/- foreseeable burden) |
To help determine where your project fits on this continuum, completion of the new Risk Assessment questions in the Human Research Ethics Application (HREA) is essential. Even if your project is considered minimal risk and exempt from review, the 2023 National Statement mandates that institutions maintain a record of these projects.
The new Risk Assessment questions address three critical requirements of the National Statement:
If your answers to the Risk Assessment questions indicate that your project is "minimal risk," you won't need to complete the full HREA. However, you must still submit a Project Description/Protocol for a full assessment, even if the project is exempt from ethics review.
In response to your feedback, we have integrated the DMP with the HREA. This integration:
Who needs to complete integrated DMP questions? Everyone completing a new HREA form for a Low or Greater than low risk project, will need to complete the DMP questions (unless you have a formal DMP form you can attach.
This unified HREA form is set to go live on August 20, 2024. Over the next few weeks leading to the new form’s release, we will provide more details and further updates including, how to guides, workshops, information videos and drop-in sessions. If you have any questions, please reach out.
Human Research Ethics Team: ethics.secretariat@mq.edu.au
Research Data Management Team: research.data.management@mq.edu.au

Friday 12 July 2024
If your work involves human subjects or their data, you need to read this.
The National Statement on Ethical Conduct in Human Research (2023) has been updated (https://www.nhmrc.gov.au/about-us/publications/national-statement-ethical-conduct-human-research-2023). One of the most significant changes is the new requirement that all human research must be reviewed for risk. This now includes research considered exempt from ethical review.
To streamline this new process and create a one-stop form for all human research, we have integrated a new Risk Assessment form AND the Data Management Plan into the HREA (Human Research Ethics Application) form.
The unified HREA form is set to go live on August 20, 2024. Over the weeks leading to the new form’s release, we will provide more details and further updates including detailed background information, help guides, information videos, workshops and times for drop-in sessions. If you have any questions, please reach out.
Human Research Ethics Team: ethics.secretariat@mq.edu.au
Research Data Management Team: research.data.management@mq.edu.au.