Tuesday 8 April 2025

Don’t forget you need to complete the "Introduction to Human Research Ethics" online training module. Over the past few months, we have been advising researchers about this mandatory requirement. It only takes approximately 30–40 minutes and provides essential knowledge on researchers' responsibilities under the National Statement on Ethical Conduct in Human Research (2023). You and all members of your research team must complete the training, or your ethics application will not be eligible for review. We do have a grace period ranging 23rd February until the 5th of April 2025 any project submitted that does not provide evidence of completion will be reviewed as normal, but the application will be sent back and approval withheld until evidence of completion is submitted. However, after this date this will change, have a look at our past communications to find out how.
How do I complete the Introduction to Human Research Ethics training module?
- For staff members: Access the training via Workday.
- For postgraduate and undergraduate students: Access the training via iLearn.
How do I provide evidence of completion once I have completed the Introduction to Human Research Ethics training module?
- There will be a question in the MQ Filter question section of the HERA on FoRA: Yes/No tick box: I confirm that ALL team members/researchers/investigators on this project have completed their “Introduction to Human Research Ethics Training module” and have included the date of completion under “Qualifications/Expertise” in the Team Members Section Q2.
- Then each team member/researcher/investigator will have to include their training module completion date under “Qualifications/Expertise” in the Team Members Section Q2.
- If you are the project owner, you will need confirm and update all team members/researchers/investigators completion date in their individual qualification section.
Thank you for your attention to this important matter. If you have any questions, feel free to reach out: ethics.secretariat@mq.edu.au
Tuesday 25 February 2025

REMINDER: You now need to complete your Mandatory Ethics Training BEFORE approval.
Don’t forget you need to complete the "Introduction to Human Research Ethics" online training module. Over the past few months, we have been advising researchers about this mandatory requirement. It only takes approximately 30–40 minutes and provides essential knowledge on researchers' responsibilities under the National Statement on Ethical Conduct in Human Research (2023). You and all members of your research team must complete the training, or your ethics application will not be eligible for review. We do have a grace period ranging 23rd February until the 5th April 2025 any project submitted that does not provide evidence of completion will be reviewed as normal, but the application will be sent back and approval withheld until evidence of completion is submitted. However, after this date this will change, scroll down to have a look at our past communications to find out how.
How do I complete the Introduction to Human Research Ethics training module?
- For staff members: Access the training via Workday.
- For postgraduate and undergraduate students: Access the training via iLearn.
How do I provide evidence of completion once I have completed the Introduction to Human Research Ethics training module?
- There will be a question in the MQ Filter question section of the HERA on FoRA: Yes/No tick box: I confirm that ALL team members/researchers/investigators on this project have completed their “Introduction to Human Research Ethics Training module” and have included the date of completion under “Qualifications/Expertise” in the Team Members Section Q2.
- Then each team member/researcher/investigator will have to include their training module completion date under “Qualifications/Expertise” in the Team Members Section Q2.
- If you are the project owner, you will need confirm and update all team members/researchers/investigators completion date in their individual qualification section.
Thank you for your attention to this important matter. If you have any questions, feel free to reach out: ethics.secretariat@mq.edu.au
Monday 17 February 2025

REMINDER: Don’t Forget to Complete your Mandatory Training for Human Ethics
Don’t forget you need to complete the "Introduction to Human Research Ethics" online training module. Over the past few months, we have been advising researchers about this mandatory requirement. It only takes approximately 30–40 minutes and provides essential knowledge on researchers' responsibilities under the National Statement on Ethical Conduct in Human Research (2023). You and all members of your research team must complete the training, or your ethics application will not be eligible for review. We do have a grace period so up until the 22 February 2025 if you have not provided evidence that all researchers on the project have completed the training your project will be reviewed as normal, and you will be sent a friendly reminder that they will need to submit an amendment within 2 months with evidence of completion or the application will be suspended. However, after this date this will change, have a look at our past communications to find out how.
How do I complete the Introduction to Human Research Ethics training module?
- For staff members: Access the training via Workday.
- For postgraduate and undergraduate students: Access the training via iLearn.
How do I provide evidence of completion once I have completed the Introduction to Human Research Ethics training module?
- There will be a question in the MQ Filter question section of the HERA on FoRA: Yes/No tick box: I confirm that ALL team members/researchers/investigators on this project have completed their “Introduction to Human Research Ethics Training module” and have included the date of completion under “Qualifications” in the Team Members Section Q2.
- Then each team member/researcher/investigator will have to include their training module completion date under “Qualifications” in the Team Members Section Q2.
- If you are the project owner, you will need confirm and update all team members/researchers/investigators completion date in their individual qualification section.
Thank you for your attention to this important matter. If you have any questions, feel free to reach out: ethics.secretariat@mq.edu.au
ALTERNATIVELY, you can register to attend one of our weekly Drop In Sessions via MyRDC https://myrdc.mq.edu.au/
Tuesday 24 December 2024

ATTENTION: Completed your Mandatory Training for Ethics submissions?
Starting NEXT MONTH, the "Introduction to Human Research Ethics" online training module will become a requirement for all researchers involved in human research. Over the past few months, we have been informing researchers of this new requirement. The module takes approximately 30–40 minutes to complete and provides crucial insights into your responsibilities under the National Statement on Ethical Conduct in Human Research (2023).
To ensure your ethics application is eligible for review, all members of your research team must complete this training. A three-month grace period will be in place to allow extra time for completion. For more details on dates and the action plan, please refer to Figure 1 below.
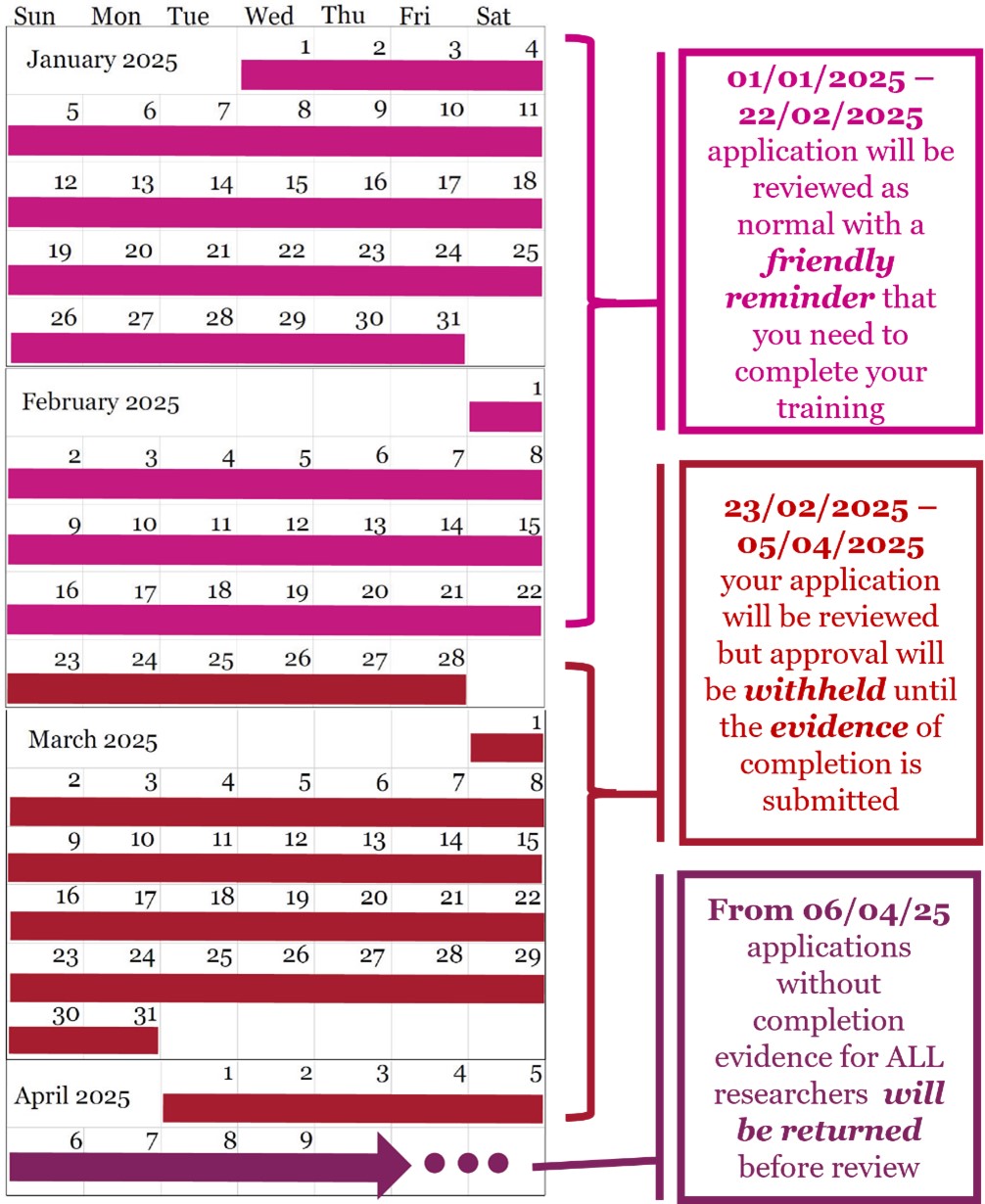
Figure 1: Three-month grace period dates and action plan.
How do I complete the Introduction to Human Research Ethics training module?
- For staff members: Access the training via Workday.
- For postgraduate and undergraduate students: Access the training via iLearn.
How do I provide evidence of completion once I have completed the Introduction to Human Research Ethics training module?
- There will be a question in the MQ Filter question section of the HERA on FoRA: Yes/No tick box: I confirm that ALL team members/researchers/investigators on this project have completed their “Introduction to Human Research Ethics Training module” and have included the date of completion under “Qualifications” in the Team Members Section Q2.
- Then each team member/researcher/investigator will have to include their training module completion date under “Qualifications” in the Team Members Section Q2.
- If you are the project owner, you will need confirm and update all team members/researchers/investigators completion date in their individual qualification section.
What will happen if I do not complete the Introduction to Human Research Ethics training module?
- Your ethics application will be returned to you after January 2025
- However, there will be a grace period (Fig. 1)
- 01/01/25 – 22/02/25 any application submitted without completion evidence will be reviewed as normal with a friendly reminder that they will need to submit an amendment within 2 months with evidence of completion or the application will be suspended.
- 23/02/25 – 05/04/25 any application submitted without completion evidence will be reviewed as normal, but the application will be sent back and approval withheld until evidence of completion is submitted.
- From 06/04/25 onwards any application without evidence of completion for all members of the research team and on the application will be returned to the researchers before going to review.
We appreciate your commitment to ensuring ethical research practices. For additional information and to review past communications, please scroll down.
If you have any questions, feel free to reach out: ethics.secretariat@mq.edu.au
Monday 16 December 2024

ATTENTION: Mandatory Training Requirement for Ethics submissions
Over the past few weeks, we have informed staff and students conducting research involving human participants about the new mandatory requirement to complete the "Introduction to Human Research Ethics" online training module. This module takes approximately 30–40 minutes to complete and provides essential knowledge on researchers' responsibilities under the National Statement on Ethical Conduct in Human Research (2023), as well as Macquarie University’s relevant policies and procedures.
At a project level, the module will assist in applying the four core ethical principles to research design and support the submission of high-quality ethics applications.
Who needs to complete the Introduction to Human Research Ethics training module?
- All Macquarie University staff and students involved in research on human participants.
- Please note that no submissions via the exempt or ethics pathways can be submitted for review without completion of the module.
When do I need to complete the Introduction to Human Research Ethics training module?
- The mandatory deadline is January 2025.
- However, there will be a grace period to allow time for completion. For specific dates, please refer to Figure 1 below.
How do I complete the Introduction to Human Research Ethics training module?
- For staff members: Access the training via Workday.
- For postgraduate and undergraduate students: Access the training via iLearn.
How do I provide evidence of completion once I have completed the Introduction to Human Research Ethics training module?
- There will be a question in the MQ Filter question section of the HERA on FoRA: Yes/No tick box: I confirm that ALL team members/researchers/investigators on this project have completed their “Introduction to Human Research Ethics Training module” and have included the date of completion under “Qualifications” in the Team Members Section Q2.
- Then each team member/researcher/investigator will have to include their training module completion date under “Qualifications” in the Team Members Section Q2.
- If you are the project owner, you will need confirm and update all team members/researchers/investigators completion date in their individual qualification section.
 Figure 1: Three-month grace period dates and action plan.
Figure 1: Three-month grace period dates and action plan.
What will happen if I do not complete the Introduction to Human Research Ethics training module?
- Your ethics application will be returned to you after January 2025
- However, there will be a grace period (Fig. 1)
- 01/01/25 – 22/02/25 any application submitted without completion evidence will be reviewed as normal with a friendly reminder that they will need to submit an amendment within 2 months with evidence of completion or the application will be suspended.
- 23/02/25 – 05/04/25 any application submitted without completion evidence will be reviewed as normal, but the application will be sent back and approval withheld until evidence of completion is submitted
- From 06/04/25 onwards any application without evidence of completion for all members of the research team and on the application will be returned to the researchers before going to review.
We appreciate your commitment to ensuring ethical research practices. If you have any questions, please don’t hesitate to contact us: ethics.secretariat@mq.edu.au
Wednesday 27 November 2024

ATTENTION: Mandatory Training Requirement for Human Research
Last week, we informed you about the new mandatory requirement to complete the "Introduction to Human Research Ethics" training module. This training is designed to assist researchers in applying the ethical principles outlined in the National Statement on Ethical Conduct in Human Research (2023), supporting both project design and the submission of high-quality ethics applications.
As previously communicated, completion of this module will become mandatory from January 2025. Please be aware that ethics applications will not be eligible for review without the successful completion of this training. To facilitate a smooth transition, a three-month grace period will be in place. However, you are encouraged to complete the module ahead of time if possible. Here's how:
- For staff members: Access the training via Workday.
- For postgraduate and undergraduate students: Access the training via iLearn.
We will provide you with more information next week but in the meantime if you have any questions, please reach out: ethics.secretariat@mq.edu.au
Tuesday 29 October 2024

ATTENTION: For ALL Research Involving Human Participants
If your work involves human participants or their data, please take note of the following important information.
To maintain the highest ethical standards in human research, Macquarie University has implemented the "Introduction to Human Research Ethics" training module, acquired in early 2024. This module is designed to provide researchers with a comprehensive understanding of their responsibilities under the National Statement on Ethical Conduct in Human Research (2023), as well as the relevant policies and procedures at Macquarie University.
Effective January 2025, completion of this module will be mandatory for all researchers conducting human research. The training will help ensure the application of ethical principles in project design and support the submission of high-quality ethics applications.
For an overview of the training module, please visit the Tricky Goose website. In the coming weeks, we will share access to the training module along with detailed information regarding the requirements for submitting ethics applications.
If you have any questions, please reach out.
Human Research Ethics Team: ethics.secretariat@mq.edu.au
Friday 16 August 2024

Integrated HREA goes live 20 August
Over the past month, we have been advising researchers on the upcoming integrated HREA form which will go live on Tuesday 20th August. The new form is essentially made up of 3 sections
- Risk Assessment Questions: to be completed if you think your project is exempt from ethics review or you don’t know or are unsure of the risk level of your project.
- Human Research Ethics Application (HREA)- to be completed if you do know the risk level of your project and you require ethics review
- Data Management Plan (DMP) Questions – to also be completed if you do know the risk level of your project and you require ethics review
Caution to updating HREA forms created before 20 August 2024
- HREAs not yet submitted for review: Recommended to SAVE A PDF of current work already completed on application, as addition of new filter question for Risk Assessment MAY cause loss of some work, i.e. tick box answers may be lost, but free text appears to be retained.
- HREAs submitted, responding to comments and not yet approved: Recommended NOT TO UPDATE form.
- HREA Amendment of approved forms: DO NOT UPDATE.
Introduction to new Exempt and Risk Assessment workflow
While the DMP and HREA sections remain largely unchanged, the 2023 updates to the National Statement introduce new requirements for risk assessment in human-focused studies. Last week, we provided MQ researchers with an overview of the relevant pathways. This week, we will introduce the new Exempt and Risk Assessment workflow (Figure 1), along with how-to guides, instructional videos, and opportunities to attend drop-in sessions (Table 1) for one-on-one support and advice.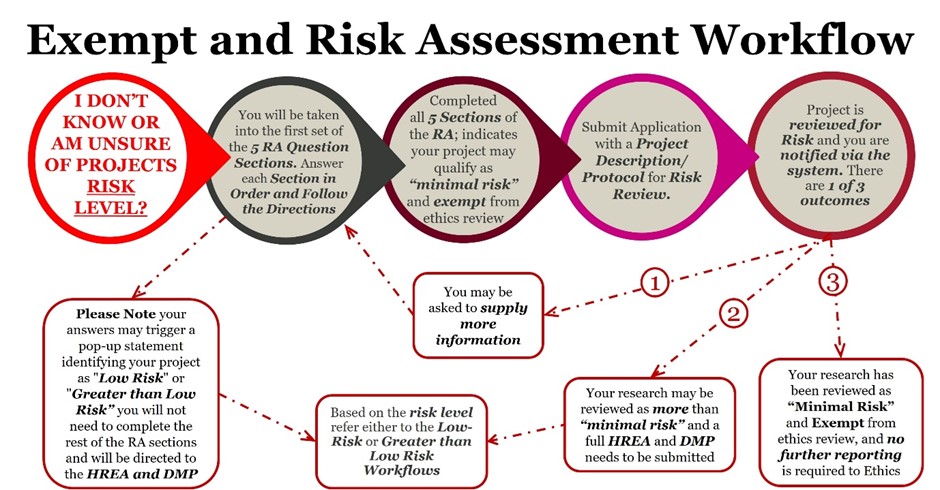
Figure1: The new Exempt and Risk Assessment Workflow – detailing the process from submission to either exemption from ethics review or determination of risk level
Guides and Drop-ins
To help you navigate your way through the new Risk Assessment and DMP filter questions of the HREA, we have created how-to-guides and instructional videos:
- Risk Assessment Section
- PDF - Risk Assessment Guide the link to the pdf is a button along the right hand side of the page
- Video: Risk Assessment and Exempt Pathway for Human Research Projects at Macquarie University
- Data Management Section
We will be holding drop-in sessions to assist with submissions of the new integrated Human Research Ethics Applications (HREA). These sessions will run from 11 am to 1 pm and are informal and flexible, allowing you to join at any time within the two-hour window and stay as long as you need. Representatives from the Ethics Secretariat and, in some cases, the Research Data Management teams will be available. Sessions will be held either in person in the Senate Room (16WW 127 Senate Meeting Room) or virtually.
Please register via MyRDC, to receive the meeting details in your calendar. Please bring your laptop if you require assistance with online submissions, as well as to access our instructional guides and videos.
If you have any questions in the meantime or would like to arrange a presentation or workshop session for your research group, please don't hesitate to reach out.
Human Research Ethics Team: ethics.secretariat@mq.edu.au
Research Data Management Team: research.data.management@mq.edu.au